Summary
This page summarizes information relevant to the sentience of various small metazoans.
Following are some crude generalizations that I've made based on the information presented in the rest of this piece. In practice, there may be significant variability among species within a given taxonomic group. The below table is a dumbed-down summary for use in back-of-the-envelope calculations.
| Taxon | Approx. number of neurons | Documented abilities |
| tunicate | ~100 | habituation/sensitization |
| turbellarian | ? | habituation, classical conditioning |
| rotifer | ~200 | habituation, sometimes precopulatory mate guarding |
| nematode | ~300 | habituation, classical conditioning |
| cladoceran | ? | habituation |
| ostracod | ≥ ~103?? | habituation, classical conditioning, maze learning, behavioral tradeoffs |
| copepod | ≥ ~103??; 300–400 for nauplius larvae? | avoidance conditioning, maze learning, sophisticated mating |
Of the animals above, my sense is that rotifers may be the least complex, based both on number of neurons (~200) and their abilities. That said, one would also expect the C. elegans nematode to be extremely simple given its number of neurons (302), yet it's capable of a variety of forms of learning, including classical conditioning and maybe even occasion setting. Perhaps the fact that I couldn't find much discussion of rotifer associative learning reflects the fact that these animals are less studied than C. elegans.
Contents
- Summary
- Tunicate
- Stenostomum (turbellarian)
- Asplanchna brightwelli (rotifer)
- Epiphanes senta (rotifer)
- Caenorhabditis elegans (nematode)
- Anguilla aceti (nematode)
- Ascaris lumbricoides (nematode)
- Daphnia magna (cladoceran)
- Cypridopsis vidua (ostracod)
- Cyclocypris forbesi (ostracod)
- Paracyclops fimbriatus poppei (copepod)
- Dactylopusia (copepod)
- Estimating neurons based on brain volumes
- Habituation results from Applewhite and Morowitz (1966)
- Aplysia sensitization
- Footnotes
Tunicate
Perry et al. (2013), pp. 567-68:
Sea squirt (tunicate) larvae, which possess only about 100 neurons, also display forms of nonassociative learning. [...] Upon light cessation, sea squirt larvae begin to swim and with light exposure they stop swimming. With repeated exposure to light, these behaviors showed both habituation (weaker responses over time), and sensitization (increased responses over time), depending on the intensity of light.
Stenostomum (turbellarian)
Classical conditioning
Applewhite and Morowitz (1967) report (p. 336):
After 4 seconds of the light, an electric shock of 5 volts and 100 msec duration in the biphasic mode was administered by a Grass stimulator; this caused Stenostomum to stop moving. The light went off after the 5 seconds, then after 25 seconds it went on again and the procedure was repeated. After each experiment, the chamber was cleaned and rotated 90° before reuse. Fifteen animals were used, and each was considered conditioned when it stopped moving four times in a row every time the light was flashed, separated by an interval of 25 seconds. It took a mean of 27.3 stimuli (SD 12.3) to condition them this way. This response was extinguished with a mean of 4.2 (SD 1.6) stimuli by presenting the 150-watt light for 5 seconds every 30 seconds until the animal no longer responded to it.
Asplanchna brightwelli (rotifer)
Number of neurons
Kotikova (1998) says (p. 139): "The brain of Asplanchna brightwelli numbers 200 cells, demonstrating a perfect bilateral symmetry (Ware & Lopresti, 1975)." I didn't see this fact mentioned when I skimmed Ware & Lopresti (1975), but this number of neurons seems plausible based on other information in Kotikova (1998), namely that across 10 rotifer species studied, "The number of the brain catecholaminergic neurons varies from 6 to 11, constituting about 3–7% of the total number of the brain cells" (p. 135).
Kotikova et al. (2005) reiterate the "200 neurons" number (p. 246): "In rotifers, brain neurons constitute 20% of the total number of cells in the body (Martini, 1912). The brain neurons of Asplanchna brightwellii number 200 cells, demonstrating a perfect bilateral symmetry (Ware & Lopresti, 1975)." Kotikova et al. (2005) also say (p. 239): "The total number of cells in the rotifer body can reach 1000 (Martini, 1912)."
This page says that rotifers "are about the same size as the larger unicellular organisms. They don't have a lot of cells, less than 1000".
Body size
This page says regarding Asplanchna brightwelli that "The females are 500 to 1500 μm long" (according to a Google Translation of the German text). Eyeballing a picture on this page confirms that the rotifer is ~1 mm long and ~1/3 mm wide.
Mating
Aloia and Moretti (1973) report (pp. 373-76):
The complete mating process of A. brightwelli requires about 45-60 sec and can be divided into four successive stages: (1) coronal contact, (2) body arching, (3) copulatory organ attachment, and (4) copulation [...]. The mating process appears to be initiated following a random encounter of a male and female. [...]
During these first two stages, requiring 10-15, sometimes 20 sec, both male and female may discontinue the courtship activity. The female may rotate around the long axis of her body or completely contract her body musculature causing the male to lose contact. The male may halt the courtship behavior simply by straightening his body and swimming away. [...]
The males of A. brightwelli have been observed to mate with other males, newborn females, and with other females two and three times their own size taken from stock mictic cultures containing vitamin E. [...] Normally, these matings have occurred between two individuals. However, occasionally, relationships such as two or three males simultaneously attached to one female, or one male copulating with one female while another male copulates with him have been observed.
Here's a video of rotifers mating (though I'm not sure what species).
Epiphanes senta (rotifer)
Here's a video of these rotifers.
Number of neurons
Applewhite and Morowitz (1966) say (p. 91): "The brain of the rotifer Epiphanes senta, for example, contains 183 neurons while the peripheral ganglia have an additional 63 neurons;3".
Precopulatory mate guarding
Schröder (2003) reports:
The females of E. senta are mostly stationary on the substrate while males are more active swimmers. If they encounter eggs with female embryos of their own species, they attend them and mate with the hatching female. Experiments showed that males are able to discriminate between male, female and diapausing eggs. They exhibit a strong preference for female eggs that are only a few hours away from hatching compared with eggs in early developmental stages. [...] It is hypothesized that males judge the age of a female egg by sensing a chemical that is produced by the growing embryo and diffuses through the egg shell.
Caenorhabditis elegans (nematode)
Number of neurons
This page says that Caenorhabditis elegans has precisely 302 neurons "In the hermaphrodite".
Eberhard and Wcislo (2011), p. 194: "C. elegans has a total of 6400 chemical and 900 gap junction synapses for 302 neurons, giving a mean of 24 synapses/cell; Altun and Hall, 2008".
Body size
This paper reports: "959 cells make up the entire adult hermaphrodite (Sulston and Horvitz 1977; Sulston et al. 1983)."
This page says that C. elegans is "about 1 mm in length". This page says that the nematode "grows to a length of 1.3 mm and a diameter of 80 μm if there is a plentiful supply of food."
Chemosensation
Bargmann (2006) says:
C. elegans has a highly developed chemosensory system that enables it to detect a wide variety of volatile (olfactory) and water-soluble (gustatory) cues associated with food, danger, or other animals. Much of its nervous system and more than 5% of its genes are devoted to the recognition of environmental chemicals. Chemosensory cues can elicit chemotaxis, rapid avoidance, changes in overall motility, and entry into and exit from the alternative dauer developmental stage.
Thermal avoidance
Nieto-Fernandez et al. (2009) explain (pp. 197-98):
The effect of morphine on the behavioral response of invertebrates to aversive stimuli is documented in the literature (Achaval et al. 2005; Barr et al. 2008; Kalil-Gaspar et al. 2007; Kavaliers et al. 1983, 1998; Lozada et al. 1988; Maldonado et al. 1989; Pryor et al. 2007; Romano et al. 1990; Romero et al. 1994). In all instances, morphine increases the latency response of the animals and opioid receptor antagonists reverse this effect. This effect of morphine on thermonocifensive behavior has also been demonstrated in the parasitic nematode Ascaris suum (Pryor et al. 2007). [...]
C. elegans exhibit thermal/warm avoidance as a survival behavior (Wittenburg and Baumeister 1999). Our results indicate that exogenous morphine, EM1, and EM2 [e]ffecta an antinociceptive response to thermal avoidance. This response is concentration dependent and is reversed by naloxone and CTOP. These findings would support the existence of an opiate-like mechanism modulating the thermonocifensive response in C. elegans.
Learning
Ardiel and Rankin (2010) report on the astounding learning abilities of an animal that has just 302 neurons:
[C. elegans] worms have been shown to habituate to mechanical and chemical stimuli, as well as learn the smells, tastes, temperatures, and oxygen levels that predict aversive chemicals or the presence or absence of food. [...]
With its invariant cell lineage and reproducible connectome, C. elegans was initially viewed as a genetically hardwired automaton that could swim forward or backward. It has since proven to be exquisitely sensitive to its environment, displaying remarkable behavioral plasticity. [...]
The deterministic development of the worm's nervous system would seem to limit its usefulness as a model to study behavioral plasticity, but time and again the worm has demonstrated its extreme sensitivity to experience—every sensory modality studied can mediate learning. [...]
Thus far, the only limit to worm learning in the laboratory seems to be the creativity of researchers in designing assays to evaluate performance.
Bargmann (2006) adds:
Learning can be context-dependent. The presence of taste cues or ethanol during olfactory learning leads to memory that is only expressed under the same taste cue or ethanol conditions (Bettinger and McIntire, 2004; Law et al., 2004). [...]
C. elegans can learn to avoid odors associated with infection by pathogenic bacteria, a behavior analogous to mammalian conditioned taste aversion (Zhang et al., 2005).
Rankin (2004) says:
studies have shown that C. elegans can learn about mechanosensory input [7], chemosensory input [8,9] and thermosensory input [10,11]. The worm can learn to approach or avoid tastes, odors or temperatures that predict the presence or absence of food. They show non-associative forms of learning, such as habituation and dishabituation, as well as associative forms of learning, such as classical conditioning and differential classical conditioning [7–11]. And they show both short-term and long-term forms of memory [7]. [...]
Law et al. [14] have now reported another sophisticated form of classical conditioning: occasion setting. In this case, the worm is hypothesized to learn a conditional relationship between stimuli: in the presence of the cues from normal growth medium, benzaldehyde predicts that no food will be present and the worm does not approach the normal growth medium. When the normal growth medium cues are not present, the worm will approach the benzaldehyde. [...]
In every area where people have looked for plasticity they have found it.
Fear-like behavior
Salk Institute for Biological Studies (2018):
C. elegans, which contains only 302 neurons, has a natural predator—another worm called Pristionchus pacificus, which bites and kills C. elegans. The researchers discovered that by exposing C. elegans to chemicals that are excreted by P. pacificus, they could elicit a fear-like response. When it encounters these predator-excreted chemicals, C. elegans rapidly reverses direction and crawls away.
They found that this fear-inducing chemical, a new class of molecules called sulfolipids, could activate four redundant brain circuits that led to this behavior. Additionally, C. elegans continued to change its behavior even after the fear-chemical was removed. [...]
“For years, we thought that only advanced brains like those of mammals would have this complex reaction,” Chalasani says. “But our study is showing that a simple animal expresses something very much like fear.”
In the experiment, coauthor and UC San Diego graduate student Amy Pribadi soaked C. elegans in a solution containing the sulfolipid for 30 minutes. The worms failed to lay eggs, even for an hour after they had been removed from the solution—an indicator of acute stress as well as a longer-term response akin to anxiety. Further research showed that the signaling pathways activated during the worms’ response are similar to the pathways activated when more complex animals experience fear.
When the worms were soaked in a solution containing Zoloft (a human anti-anxiety drug), however, these fear- and anxiety-like responses were not observed.
Male mating
Barr and Garcia (2006) report:
In a stereotyped mating event [...], the male initially responds to hermaphrodite contact by placing his tail flush on her body; he begins moving backwards along her body until he reaches her head or tail, where he then turns via a sharp ventral coil. He continues backing until his tail contacts the vulva; at that region of the hermaphrodite, he stops moving, inserts his spicules, and ejaculates into the hermaphrodite uterus. Completion of all sub-behaviors is not mandatory for successful copulation. For example, if the initial contact is on the ventral side of the hermaphrodite, the male may immediately locate the vulva, insert his spicules, and ejaculate without evoking turning behavior.
You can see the mating steps here.
Videos
Anguilla aceti (nematode)
I think these are now known as Turbatrix aceti, which "may be found in unfiltered vinegar."
Number of neurons
Applewhite and Morowitz (1966) say (p. 91): "the nematode Anguilla aceti has a nervous system of 279 nerve cells.10"
Ascaris lumbricoides (nematode)
Number of neurons
Eberhard and Wcislo (2011) report (p. 174) that the number of neurons is "approximately 254 in the nematode Ascaris lumbricoides (Bullock and Horridge, 1965)".
Daphnia magna (cladoceran)
Number of neurons
 Sims and Macagno (1985) created a computer reconstruction "of all of the approximately 200 neurons in one half of the bilaterally symmetric adult optic ganglion of the small crustacean Daphnia magna." That means the full optic ganglion, counting both halves, would have ~400 neurons. Obviously, this is less than the full number of neurons in the whole animal, but I'm not sure how much less. (I didn't read the full Sims and Macagno (1985) article, but I doubt it gave this information.)
Sims and Macagno (1985) created a computer reconstruction "of all of the approximately 200 neurons in one half of the bilaterally symmetric adult optic ganglion of the small crustacean Daphnia magna." That means the full optic ganglion, counting both halves, would have ~400 neurons. Obviously, this is less than the full number of neurons in the whole animal, but I'm not sure how much less. (I didn't read the full Sims and Macagno (1985) article, but I doubt it gave this information.)
Body size
This page says "The females reach up to 5 mm in size, the males about 2 mm, and thus being among the largest Daphnia species.[5]"
Cypridopsis vidua (ostracod)
Body size
This page shows a female Cypridopsis vidua with a length of 0.6 mm.
Behavioral tradeoffs
See "Ostracod Food vs. Antipredator Tradeoff".
Cyclocypris forbesi (ostracod)
Body size
Applewhite and Morowitz (1966) say that this species is 0.6 mm (p. 103).
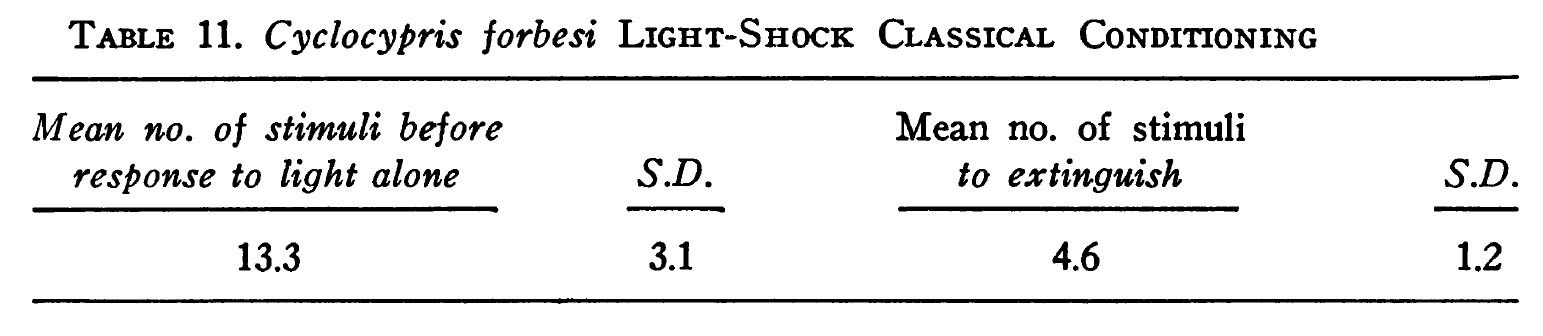
Classical conditioning
Applewhite and Morowitz (1966) report (p. 103) classical conditioning in Cyclocypris forbesi by pairing light with electric shock. The shock "caused the ostracod to stop moving about in the chamber and close its shell." And "The animals were considered conditioned when the light alone presented every 115 seconds caused them to close their shells three times in a row." The results were as follows:
Maze learning
Applewhite and Morowitz (1966) had Cyclocypris forbesi proceed through a series of chambers. The hole to the next chamber was either always on the left side or always on the right side. Hence, the ostracod could learn over time where the hole was likely to be, allowing it to move through the chambers faster. As a control, the position of the hole was alternated left vs. right, which was "a more difficult learning task" (p. 100). Applewhite and Morowitz (1966) report (p. 100):
The results [...] for the experimental groups indicate the time spent in each chamber decreases with the number of trials. [...] For the control groups, there was no significant change in performance among the seven chambers used or the retention period afterward [...].
Paracyclops fimbriatus poppei (copepod)
Body size
Applewhite and Morowitz (1966) report that this species is 0.8 mm (p. 97).
Avoidance conditioning
Applewhite and Morowitz (1967) showed avoidance learning in Paracyclops fimbriatus poppei (p. 337):
for the experimental group, a shock of 2.5 volts for 100 msec duration in the biphasic mode was given by a Grass stimulator every time the animal went in the dark during the next 3 minutes. No more than two shocks were given to the animal when it was in the dark, and an average of 30 shocks were given over the 3-minute period. Then, the time spent in the dark by each animal for the next 2 minutes was recorded. [...] For the experimental group, the animals spend less time in the dark side of the chamber after they are shocked for going into that side.
For some reason, using the same procedure but shocking the copepods only when they were in the light part of the chamber, rather than only when they were in the dark part, didn't lead to learning (p. 337).
Maze learning
Applewhite and Morowitz (1966) report that Paracyclops fimbriatus poppei could learn in the same maze test as was discussed above for Cyclocypris forbesi. In fact, the authors say (p. 100): "the copepods are exhibiting one-trial learning[...]. There is some controversy over
whether animals are capable of such noncontinuity (nongradual) learning, but there are examples of it.19"
Dactylopusia (copepod)
Number of neurons
Eberhard and Wcislo (2011) report (p. 174) that the number of neurons is "perhaps 300–400 and certainly less than 1000 in the nauplius larva of the copepod Dactylopusia (T. Lacalli, personal communication)." I wonder if adults have more neurons?
Estimating neurons based on brain volumes
Applewhite and Morowitz (1967) include the following helpful table (though they don't specify where these numbers come from):
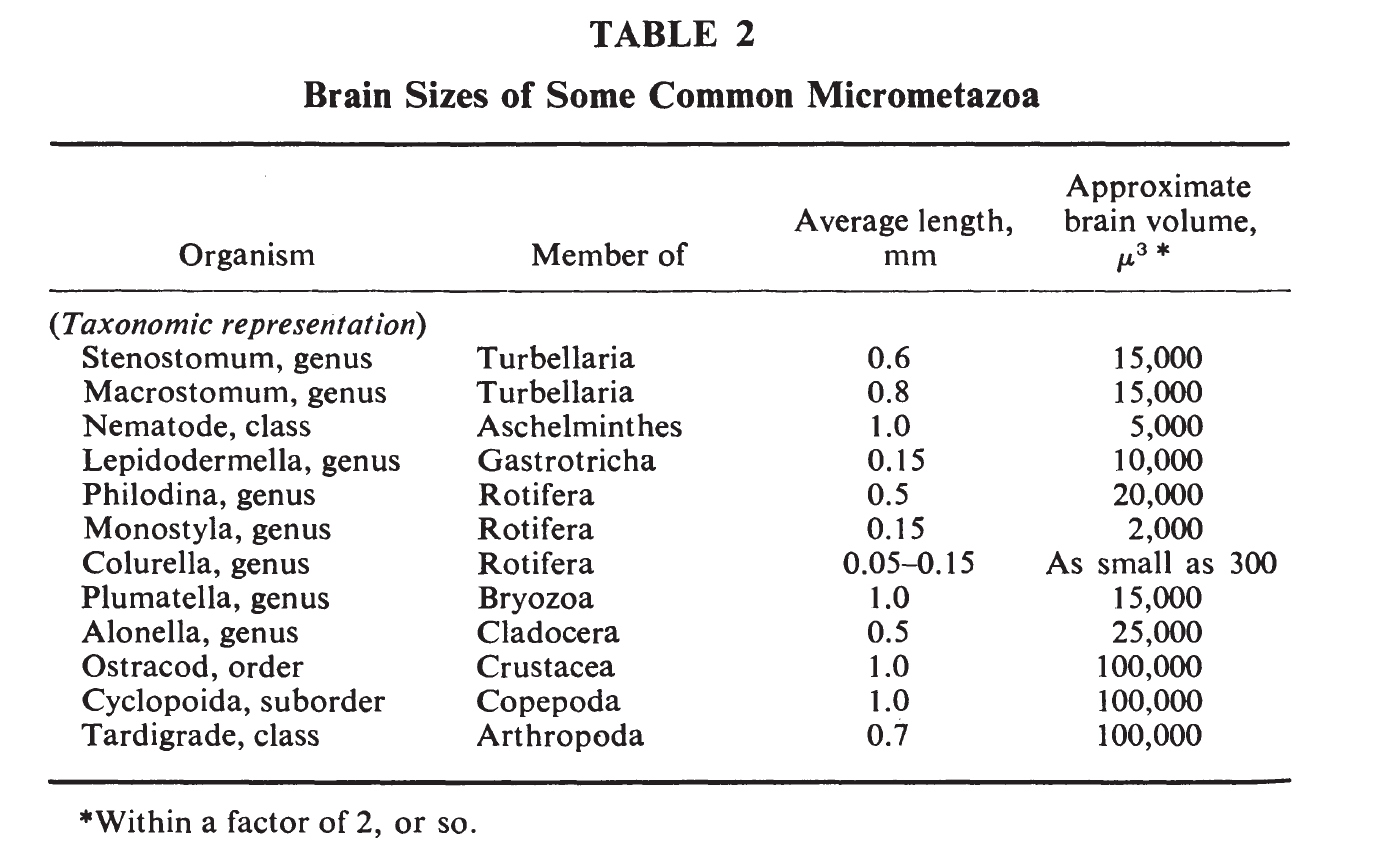
I think μ3 means μm3.b
As mentioned above, Asplanchna brightwelli females are 0.5 to 1.5 mm long. Of the rotifers in the above table, Philodina comes closest in length, at 0.5 mm. Assume that Philodina, like Asplanchna brightwelli, have ~200 neurons. Given a brain volume of 20,000 μm3, Philodina would then have roughly 200 / 20,000 = 0.01 neurons per μm3 of brain volume. Based on that ratio, we would expect ostracods, cyclopoids, and tardigrades to have roughly ~1000 neurons, although this estimate could easily be off by quite a bit.
An estimate based on nematode neurons would yield higher numbers. The average length of nematodes in the above table is 1 mm, the same as for C. elegans. (302 neurons) / (5000 μm3) = 0.06 neurons per μm3.
Applewhite and Morowitz (1966) explain (p. 91):
From the standpoint of small size, members of the rotifer genus Colurella are particularly interesting since their minimum size of 50 μ makes them the smallest metazoan.9 The brain is no larger than 500 μ3, probably contains less than 200 neurons, and has a dry weight of the order of 10-9 grams.
If the brain does contain 200 neurons, this would imply a whopping 200 neurons / (500 μm3) = 0.4 neurons per μm3.
Another estimate of neurons per unit volume comes from the following passage in Applewhite and Morowitz (1966), p. 90:
Recently, the planarians have received considerable attention as a model system that is much less complex than that of mammals. Their dimensions are of the order of centimeters in length and the brain the order of one cubic millimeter. Although planarian neurons are smaller than mammalian ones, their brain still contains the order of 105 cells.
The brain volume is ~1 mm3 = 109 μm3, which implies only 0.0001 neurons per μm3. Perhaps planarian neurons are bigger than those of smaller animals?
Applewhite and Morowitz (1966) also note (p. 91):
the rotifer Monostyla and gastrotrich Lepidodermella, both less than 150 μ in length, have brains smaller than one nerve cell body from the spinal ganglion of a dog or Purkinje cell of a cat.
That said, Purkinje cells "are some of the largest neurons in the human brain", so I don't know how representative this comparison is.
Habituation results from Applewhite and Morowitz (1966)
Applewhite and Morowitz (1966) showed habituation for many kinds of small creatures in response to "mechanical shock", by which they mean a physical disturbance caused by "the jar of the ringstand hitting the table" (p. 92). The authors reported (p. 93):
To this kind of mechanical stimulus, the protozoan Spirostomum (length 1 mm.), the flatworms Stenostomum (.6 mm.) and Macrostomum (.8 mm.), the rotifer Philodina (.4 mm.) and the bryozoan Plumatella (1 mm.) contracted; the rotifers Monostyla (.13 mm.) and Colurella (0.7 mm) and the gastrotrich Lepidodermella (.15 mm.) stopped moving; the cladoceran Alonella (.5 mm.) and the ostracod Cyclocypris forbesi (.6 mm.) closed their shells.
All of these organisms were shown to habituate (pp. 94-97).
Aplysia sensitization
The Aplysia gill and siphon withdrawal reflex offers a classic example of habituation and sensitization. The basic machinery underlying the processes is fairly well understood and seems relatively simple compared with most neural algorithms.
Sometimes the example of Aplysia is raised to claim the absurdity of the idea that simple behaviors like sensitization could indicate (any degree of) consciousness. For example:
In fact, the [gill and siphon withdrawal] GSW reflex model that led to Eric Kandel’s Nobel prize can be replicated by repetitive puffing of 5HT onto a single neuron. Would you consider this individual neuron be “sentient”?
First of all, the neural system involved is far more than one neuron. This video explains (at 2:50): "Although hundreds of neurons are ultimately involved in producing this simple behavior, the activities of only a few different types of neurons can account for gill withdrawal during habituation and sensitization." And this larger neural context is important for giving meaning to the biochemical changes that occur within a single neuron. As an analogy, a critic could belittle the idea of pain in humans by asking whether merely stimulating peripheral pain neurons is "conscious suffering". But we should be looking at the whole context of what those pain neurons do within the nervous system, not just those neurons in isolation. This is similar to the "systems reply" to the Chinese Room argument.
Second, I would argue that even if the machinery is very simple, the behavior can still have traces of moral importance, assuming we care about functional behavior rather than how many neurons you have for the sake of having neurons. At a high level, sensitization is vaguely redolent of a familiar human experience of "becoming jumpy" after getting scared or shocked. One event can set you off and make you more reactive to other events. In the human case, this behavior comes along with a vast suite of other neural and somatic changes, including probably some self-reflective thoughts about how you've become more anxious. In Aplysia, sensitization involves vastly fewer of these changes. But Aplysia sensitization still captures an outline of the process that we consider morally relevant in the human case, and I don't see a reason to regard Aplysia sensitization as morally completely irrelevant (rather than just much less important) merely because it's much simpler and doesn't capture most of the nuance that human jumpiness has.
Footnotes
- The original spelling of this word was "affect". I think, but am not sure, the authors meant "effect", i.e., "bring about". (back)
- Here are sanity checks on some of the numbers in the table, based on the observation that brain-to-body mass ratios typically fall within the range of ~1/10 to ~1/1000:
- This page says that Philodina are "Up to 500 micrometers in length when extended", which agrees with the "Average length" value in the Applewhite and Morowitz (1967) table. The picture on this page shows that these rotifers are much longer than they are wide. Let's guess that they're, say, ~6 times longer than wide. Approximating a rotifer as a rectangular box, the body volume is (500 μm) * [(1/6) * (500 μm)] * [(1/6) * (500 μm)] = 3 * 106 μm3. Compared against a brain volume of 20,000 μm3 in the above table, this implies a brain-to-body-mass ratio of 1/174 (assuming that mass is proportional to volume, i.e., that brain and body densities are the same). There are huge error bars on this brain-to-body-mass estimate, but at least it seems to be in a plausible ballpark.
- The table lists nematodes as being 1 mm long, which roughly agrees with this quote from earlier in my piece regarding C. elegans: "grows to a length of 1.3 mm and a diameter of 80 μm if there is a plentiful supply of food." Let's approximate a nematode as a cylinder 1 mm long and (80 μm) * (1/1.3) ≈ 60 μm in diameter. Then the body volume is (1000 μm) * π * (30 μm)2 = 3 * 106 μm3. Compared against a brain volume of 5000 μm3, this gives a brain-to-body-mass ratio of 1/565.
- The table lists ostracod lengths as 1 mm. Suppose the width and height are, say, half that much: 500 μm. (This seems somewhat plausible based on pictures of ostracods.) Then the volume is roughly (1000 μm) * (500 μm) * (500 μm) = 2.5 * 108. This implies a brain-to-body-mass ratio of 1/2500, which seems low but isn't crazy.